The Fifth Kingdom - Chapter 2a
PROTOZOAN 'PSEUDOFUNGI'
the so-called 'Slime Moulds' -- -- Phyla MYXOSTELIDA, DICTYOSTELIDA,LABYRINTHULIDA, and PLASMODIOPHORIDA
Click on any of the four group names to go to its section of the chapter
First let me elaborate a little on the definition of fungi given earlier. Fungi (whether chromistan or eumycotan) are heterotrophic (non-photosynthesizing) eukaryotes that absorb their food, typically at the many growing points of their rather diffuse, indefinite `body' (often called a thallus or mycelium), which is made up of fine branching tubes called hyphae. The wall of the tubes is mainly composed of chitin or cellulose, and within this wall the cytoplasm and nuclei live and move, protected from the outside world, but able to explore small areas of it inside their apically extending, microscopic, hyphal tunnels, and much larger areas by means of their detachable reproductive units called spores. But before I discuss real fungi, four 'outsider' phyla must be mentioned. These are the so-called 'slime-moulds,' the inappropriately named Myxomycota, Acrasiomycota , Labyrinthulomycota and Plasmodiophoromycota. (because none of them are fungi)Here's a short quotation from a fascinating essay, 'Either or Neither', by David Quammen:
"They aren't animals, they aren't plants, they aren't fungi. They aren't single-celled organisms (except on a part-time basis) and they aren't (except part-time) multicellular... They're neither this nor that, but always something else -- something whose identity is protean. They flow like spilled syrup across the ruler-straight lines of binary definitude... They shift shape, according to circumstance and mood. They transmogrify spookily."
(see references)
They're slimy, but they're not mouldy! Although they used to be thought of as fungi, and are still dealt with in some current mycological literature, this is at least phylogenetically incorrect. Here's why. The assimilative or somatic phases of three of these four groups are basically amoeboid: none of the four ever produces hyphae (a diagnostic feature of the vast majority of true fungi), and the assimilative plasmodia don't even have cell walls. The names currently applied to some of these groups are misleading, in that they imply a fungal nature, so in three cases I have supplied new names reflecting their non-fungal affiliations, which lie in Kingdom Protozoa. I hope you will agree with me after you have read the thumbnail sketches below and compared them with my later descriptions of the wall-possessing hyphal fungi. These phyla are included in some mycology courses because some of them, particularly the myxomycetes [=Myxostelida], tend to turn up when we look for fungi. And if mycologists, who have historically looked after them, abandon them, which other group of organismic biologists will agree to add these organisms to their already crowded course schedules? None, we suspect, which is why they appear here.
This is the only one of these four non-fungal phyla you are likely to find if you go out looking for fungi in Autumn. The macroscopic, slimy, amoeboid plasmodium (the somatic or assimilative phase of the organism), populated by diploid nuclei, oozes around in the soil or in
decaying wood or other organic matter, eating (yes, actually engulfing or ingesting) bacteria and other tiny food particles. I found this one in the stump of a banana plant.
You can keep the plasmodia of some myxostelids as pets (as many of my students did with Physarum polycephalum), crawling around in petri dishes and eating particles of old-fashioned rolled oats (they don't like instant oats nearly as well).
The plasmodium in the photograph below has exhausted its small central food supply, and is now fanning out all over the surface of the agar looking for more food.
Even when plenty of food is available this will not go on indefinitely...eventually the slowly pulsating plasmodium will metamorphose dramatically, as this one of P. polycephalum (below) is doing...



Spore formation involves reduction division (meiosis), so when each haploid spore later germinates to release a cell that may be either amoeboid (as in the picture below) or biflagellate, this cell acts as a gamete.
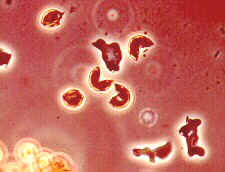


The tall brown mature sporangia (above, left) are those of Stemonitis fusca (order Stemonitales). On the upper right is a highly magnified view of the non-cellular capillitium and the dark columella that runs up the centre of the sporangium. The picture below is of a particularly massive fruiting of this organism that erupted in a rotting trailer. Spectacular, and no doubt unnerving for the owner...

I'd also like to share with you a collection I and my twin granddaughters made on the 15th July 2005. It is far less conspicuous than the one shown above, but the plasmodium must have been quite successful in its food-gathering activities, because it produced literally hundreds of (albeit tiny) sporangia. The myxostelid was Arcyria cinerea and I was able to record some microscopic as well as macroscopic details, as the sequence of five pictures below will show you. The last two show spores and ornamented non-cellular capillitium.





Although I have so far shown you only regular-style sporangia, there are actually four different kinds of myxomycete fruiting body, though these are not used to separate the six orders.
(2) The aethalium is a cushion-shaped, sessile structure. Aethalia are groups of fused sporangia (they have lost their separate peridia) and can be several centimetres across.
(3) The pseudoaethalium (which means false aethalium). This is a mass of individual sporangia, with individual peridia, but developing in a tight cluster. Pseudoaethalia are usually sessile.
(4) The plasmodiocarp which is net-like, resembling a frozen plasmodium.
Look for examples of these four kinds among the pictures that follow the key.
Key to the Orders of Myxostelida
(1) Spores produced singly on individual hair-like stalks.......Ceratiomyxales
(Example: Ceratiomyxa)
(1) Spores produced in large numbers inside sporangia of various shapes and sizes.......2
(2) True, non-cellular capillitium threads among spores (often ornamented).......3
(2) True capillitium absent (wide pseudocapillitium may be present).......Liceales
(Examples: Lycogala, Tubifera, Dictydium, Cribraria)
(3) Spores light or brightly coloured.......4
(3) Spores darkly pigmented.......5
(4) Sporangia sub-microscopic (smallest known Myxostelids).......Echinosteliales
(Examples: Echinostelium, Clastoderma)
(4) Sporangia larger or aggregated in various ways.......Trichiales
(Examples: Trichia, Arcyria, Hemitrichia, Metatrichia)
(5) Calcium carbonate deposits absent.......Stemonitales
(Examples: Stemonitis, Comatricha, Lamproderma)
(5) Calcium carbonate deposits on and in fruiting bodies.......Physarales
(Examples: Physarum, Fuligo, Diachea, Didymium, Badhamia)
Echinostelium (order Echinosteliales) is much smaller, as you may guess from the tiny amount of capillitium present in the sporangium shown (below).


The net-like plasmodiocarp of Hemitrichia serpula (order Trichiales - above, left) retains the outline of the plasmodium; its light coloured spores and true capillitium (solid, non-cellular threads with a helical ornamentation - above, right - not to be confused with hyphae) place it unequivocally in the order Trichiales.



Then common Fuligo septica (below) and its aethalium also belong to the Physarales. The peridium contains lime, and the spores are very dark

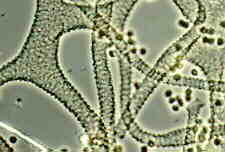
The very common aethalia of Lycogala epidendrum (above, left) may also represent a number of fused sporangia. It has pale spores and a pseudocapillitium (above, right) that place it in the order Liceales. My thanks to Prof. George Barron for several of the photos above.
Here is what most people would regard as an insoluble mystery. What is it?

Another common, if atypical, myxostelid is Ceratiomyxa fruticulosa, of the order Ceratiomyxales, in which the sporangia are extremely small and contain only one spore. Sporangia are produced simultaneously all over the surface of white fructifications: my picture shows these before the sporangia develop

The picture of Ceratiomyxa below was taken by Ray Simons, and I encourage you to visit his web-site, which illustrates a wide variety of slime moulds:



Phylum DICTYOSTELIDA (formerly ACRASIOMYCOTA)
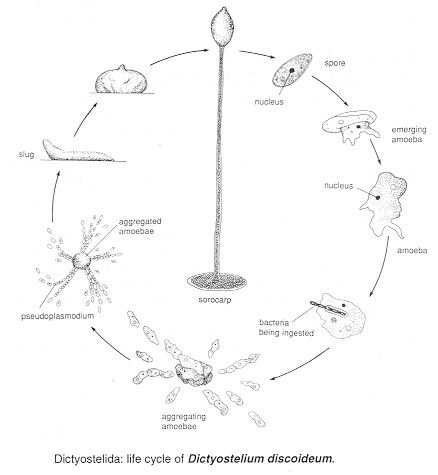

Now its DNA has been fully sequenced, and 97 authors (Eichinger et al. 2005) have found that "The gene-dense chromosomes...encode approximately 12,500 proteins, a high proportion of which have long, repetitive amino acid tracts. There are many genes for polyketide synthases and ABC transporters. The genome is rich in complex repeats, one class of which is clustered and may serve as centromeres. Partial copies of the extrachromosomal ribosomal DNA (rDNA) are found at the ends of each chromosome, suggesting a novel telomere structure and use of a common [shared] mechanism to maintain both the rDNA and chromosomal termini. A proteonome-based phylogeny shows that the amoebozoa diverged from the animal-fungal lineage after the plant-animal split, but Dictyostelium seems to have retained more of the diversity of the ancestral genome than have plants, animals or fungi."
My second example of this strange phylum is Polysphondylium pallidum. Basically amoeboid during its growth phase, it produces a sorocarp that mimics the hyphomycete, Verticillium (see Chapter 4a), as the illustration below shows. Note that the outlines of the individual amoebae can still be seen after the branched sorocarp has developed (also note that these are not hyphae, and the lines are NOT septa). The 'cells' of the stipe and branches die: only those that reach tips and form the spores survive. Is this a form of altruism?
Phylum LABYRINTHULIDA (formerly LABYRINTHULOMYCOTA)
Zostera effectively disappeared from the Atlantic coasts. This led to serious declines in waterfowl populations and in scallop fisheries. But neither Zostera nor those other species died out: Zostera could tolerate a much wider range of salinities than Labyrinthula, so it was able to hang on in brackish water. But a limpet species, Lottia alveus, which lived ONLY on eelgrass, could NOT tolerate reduced salinity, so could not follow its food plant. While the eelgrass survived in brackish waters, Lottia had lost its special habitat, and died out. When the eelgrass recovered, Lottia was gone. It is interesting that humans played no part in this particular extinction, though we have been guilty in many (most?) other subsequent examples!
The spindle-shaped, naked cells of the colony live and move entirely within a network of narrow, tubular, polysaccharide sheaths which they themselves secrete. They release biflagellate gametes, and the zygote divides mitotically to generate a new colony, whose cells are presumable diploid. Most of the other 10 members of this group are also marine, parasitizing algae. However, a new species, L. terrestris, has recently been found causing 'rapid blight' and death of turf grasses in in California and nine other states (Bigelow et al. 2005).
The picture below is an SEMicrograph of an aggregation of amoebae of L. zosterae within a sheath. The lower picture shows a network.


Phylum PLASMODIOPHORIDA (formerly PLASMODIOPHOROMYCOTA)
All members of this group are obligate parasites. Plasmodiophora brassicae produces uninucleate, biflagellate primary zoospores which penetrate a root hair of its host, cabbage (Brassica oleracea). Inside, they grow into multinucleate but still microscopic primary plasmodia. These eventually develop a wall and divide internally into uninucleate secondary sporangia. These germinate, releasing four secondary, biflagellate zoospores which leave the host. These may also act as gametes, fusing in pairs, but soon infect a root again, developing within host cells into multinucleate secondary plasmodia. At maturity, these can cleave into uninucleate cysts, each containing a single spore, which can persist in the soil for many years. This parasite stimulates the cabbage roots to become grossly swollen, a serious disease condition known as "club root" (shown below).


The four groups just outlined are all clearly non-fungal members of the Kingdom Protozoa, but the current arrangement of phyla within that kingdom places them in three rather different categories. The Dictyostelida is one of four phyla which lack flagella, but have complex sexual cycles (group II). The Labyrinthulida and Plasmodiophorida are among fourteen phyla which display reversible formation of flagella, and lack complex sexual cycles (group III); while the Myxostelida and eleven other phyla display reversible formation of flagella, and exhibit complex sexual cycles (group IV).
If you would like to read a fascinating, and beautifully written, essay about slime moulds, may I recommend "Either or Neither", pages 139-149 in The Boilerplate Rhino - Nature in the eye of the beholder by David Quammen. Your local library probably has it (mine did). Many of the other essays, all relatively short, also make stimulating reading.
Go to Chapter 2b Go to Table of Contents© Mycologue Publications 2020
Further Reading
Barron GL (1991) Protoplasm in motion. Seasons 31(2): 20-25.
Bigelow DM, Olsen MW, Gilbertson RL (2005) Labyrinthula terrestris sp. nov., a new pathogen of turf grass. Mycologia 97: 185-190.
Bonner JT (1967) The Cellular Slime Molds. 2nd Edn. Princeton University Press, Princeton.
Bonner JT (1993) Life Cycles - reflections of an evolutionary biologist. Princeton University Press, Princeton.
Cavender JC (1990) Phylum Dictyostelida. pp. 88-101 (in) Handbook of Protoctista (eds) Margulis L, Corliss JO, Melkonian M and Chapman DJ. Jones and Bartlett, Boston.
Dylewski DP (1990) Phylum Plasmodiophoromycota. pp. 399-416 (in) Handbook of Protoctista (eds) Margulis L, Corliss JO, Melkonian M and Chapman DJ. Jones and Bartlett, Boston.
Eichinger L et al.[93 authors!] (2005) The genome of the social amoeba Dictyostelium discoideum. Nature 435: 43-57.
Frederick L (1990) Phylum plasmodial slime molds: class Myxomycota. pp. 467-483 (in) Handbook of Protoctista (eds) Margulis L, Corliss JO, Melkonian M, Chapman DJ. Jones and Bartlett, Boston.
Gray WD, Alexopoulos CJ (1968) Biology of the Myxomycetes. Ronald, New York.
Hagiwara H (1989) The taxonomic study of Japanese Dictyostelid slime molds. National Science Museum, Tokyo.
Margulis L, Corliss JO, Melkonian M, Chapman DJ (eds) (1990) Handbook of Protoctista. Jones and Bartlett, Boston.
Muehlstein LK, Porter D, Short FT (1991) Labyrinthula zosterae sp. nov., the causative agent of wasting disease of eelgrass, Zostera marina. Mycologia 83: 180-191.
Olive LS (1975) The Mycetozoans. Academic Press, New York.
Porter D (1990) Phylum Labyrinthulomycota. pp. 388-398 (in) Handbook of Protoctista. (eds) Margulis L, Corliss JO, Melkonian M and Chapman DJ. Jones and Bartlett, Boston.
Quammen D (2000) The Boilerplate Rhino: Nature in the Eye of the Beholder. Scribner, New York.
In Google, type in: YouTube videos slime molds. Note that different phyla will be illustrated, and please do not accept text or labels without checking. However, some of the time-lapse sequences you will find are very good.
