The Fifth Kingdom - Chapter 11
FUNGAL ECOLOGY
Hotlinks to: the dung succession - amphibious
fungi in streams
aero-aquatic fungi in ponds - pine needle
succession
fire fungi - macrofungal ecology
- fairy rings - the humungous
fungus -
ATBI (All Taxa Biodiversity Inventory)
The Succession of
Coprophilous Fungi
The first habitat is dung (which is also known by many other names). We may turn up our noses, but to some other organisms, dung is a considerable resource, which is constantly being produced in large quantities by billions of animals all over the world. You may think that because it has passed through an animal's digestive tract, every bit of nutritional value will have been extracted from it. This is a false impression. There may not be a lot of high quality protein left, but there is a great deal of microbial biomass, as well as many food components, for example, cellulose, that neither the animal nor its gut flora managed to digest. There are also excretory products which, though they are of no further value to the animal, are high in nitrogen: herbivore dung may contain 4% nitrogen -- more, in fact, than the plant material originally eaten by the animal. So, at frequent intervals throughout its life, every mammal evacuates from its gut a mass of first class fungal substrate, simply asking to be exploited.
Are there fungi which specialize in exploiting dung? And if there are, how do they gain
access to this substrate when it becomes available? The answers may surprise you. About
175 genera of ascomycetes are largely or exclusively found on dung. The extremely advanced
and successful agaric genus Coprinus has many species that occur exclusively on
dung. There are also many specialized dung-inhabiting zygomycetes, among which Pilobolus
and some of the elaborate anamorphs in the order Kickxellales are perhaps the most
spectacular. So there is no doubt that a specialized mycota of dung-inhabiting (coprophilous)
fungi exists.
But how do they compete successfully for this substrate? The answer here may be a little
unexpected, but it is nevertheless perfectly logical. These fungi contrive to be first to
exploit the dung by the simple expedient of being in it when it is
deposited.
The only way to achieve that is to be eaten by the animal.
Coprophilous fungi manage this trick in several ingenious ways. These processes must
take into account some immutable logic. 1) The fungi are growing in the dung and will
therefore have to fruit on it. 2) Animals do not, in general, eat their own dung (though
rabbits do, raising interesting questions about the coprophilous fungi associated with
them). 3) Therefore, the spores must be somehow distanced from the dung in such a way as
to increase their likelihood of being eaten by herbivorous mammals.
You have already read in earlier chapters about how several fungi of herbivore dung
achieve this trick. How the zygomycete, Pilobolus, aims and shoots its sporangia
up to 2 metres toward the light.

(photo above by W.R.West)


How the ascus tips of the apothecial ascomycete, Ascobolus, protrude from the
hymenium and bend toward the light before shooting their spores.

How the necks of the perithecial ascomata of Podospora and Sordaria bend
toward the light before their ascospores are expelled.



Each of these independently evolved phototropic
mechanisms is obviously designed to direct the spores away from any other adjacent dung,
and to increase the efficiency with which spores are deposited on nearby vegetation that
has a good chance of being eaten by the animal.
Many other dung-inhabiting fungi are less specialized than those I have just mentioned, or
have specializations so subtle that we have not yet detected them. Nevertheless, the fact
remains that with patient and repeated examination, we can find a large number of fungi
representing most of the major fungal groups on the dung of many herbivorous mammals.
Repeated
observations will show that the various fungi tend to sporulate in a reasonably definite
sequence. First the Zygomycetes
will appear...Pilobolus [above, right], and other
saprobic genera such as Mucor, Phycomyces, Thamnidium,
Helicostylum and others . Then the dichotomously branched sporangiophores of Piptocephalis
[bottom, right] which is parasitic on
some of the zygomycetes just mentioned...as
are Chaetocladium and Syncephalis [bottom, left].

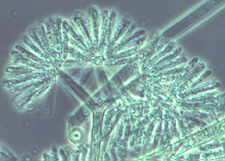
The tall sporangiophores of Syncephalis [below] with their swollen apices and
linear merosporangia...

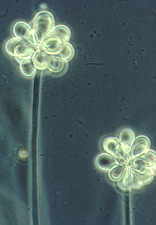
The graceful multiple recurved sporangia of Circinella minor (the three
pictures below show a developmental sequence...note the columellas in the
third picture.
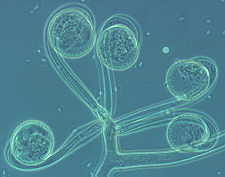
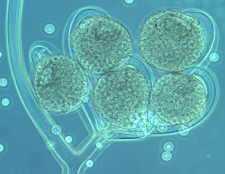
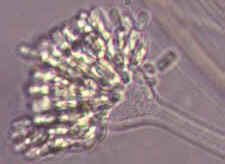
Rhopalomyces elegans [below], which parasitizes nematode eggs...

Cunninghamella with its apical vesicle and unispored sporangia...


Then the Ascomycetes ..apothecial
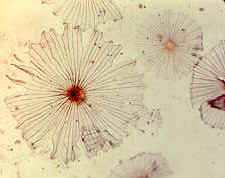
fungi like Ascobolus...zooming in from left to right...



Saccobolus...again zooming in from left to right
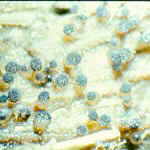


Thecotheus...here with 32-spored asci


And the perithecial Podospora and Sordaria,
accompanied by a variety of conidial anamorphs (Hyphomycetes) such as the
blastic-sympodial Basifimbria...


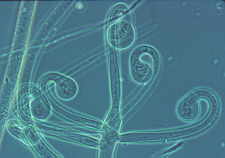
...the nematode-trapping Arthrobotrys with its clustered didymosporous
(2-celled) conidia
and its various ways of snaring nematodes, including the 3-dimensional net shown here
[below, right]...


the synnematal, slimy-spored (arthropod-dispersed) Graphium, here shown in a
zoom sequence of four pictures...




the synnematal, dry-spored Cephalotrichum
(bottom, right)...


...and Trichurus, a synnematal hyphomycete with twisted, hair-like
setae arising all over the fertile head, which give
it a 'big hair' look (below)...

and finally the Basidiomycetes,
mainly small (but profuse) species of Coprinus...





It has been suggested that this is a true ecological succession, albeit a miniature
and condensed one. Initially it was postulated that the sequence was a nutritional one.
Zygomycetes can generally assimilate only fairly accessible carbon sources, such as
sugars. Their fast growth was assumed to give them an advantage in finding these, and
their early disappearance was thought to be due to the exhaustion of this substrate. The
ascomycetes and conidial anamorphs that appeared next were assumed to be able to
assimilate more complex carbon sources such as hemicellulose and cellulose; while the
basidiomycetes, appearing last and persisting longest, were able to exploit both cellulose
and lignin.
But when this hypothesis was scrutinized more carefully and tested by
experiment and further observation, it did not hold up. The growth rates of the various
fungi were found to be relatively similar, and the various carbon sources were not
exhausted as quickly as had been assumed. So a second hypothesis was advanced. This one
was based on the time it took for each kind of fungus to accumulate enough food reserves
to permit it to fruit. It was argued that the simple sporangiophores of the zygomycetes
could be developed after only a short period, while the more elaborate fruit bodies of the
ascomycetes would require a longer build-up, and the even larger basidiomata of the
coprini would need the longest preparation of all. This is a more reasonable hypothesis,
because if we grow some of the dung fungi on laboratory media, we find that it takes Mucor
hiemalis 2-3 days to sporulate, while Sordaria fimicola needs 9-10 days, and
Coprinus heptemerus 7-13 days.
Some of the Kickxellales, zygomycetes often found on the dung of sedentary mammals
(those with a defined home base, a small territory, and habitually used paths) produce
extremely complex and convoluted anamorphs.
Spirodactylon, possibly the most
complex of all, produces tall, branched sporangiophores that bear tiny coils within which
develop innumerable one-spored sporangia. The whole structure must be designed to catch on
the hairs of the rat or mouse as it passes by. This is made possible by the habits of the
animal which, although it doesn't eat its own dung, at least deposits it somewhere along
one of the trails it follows every day in its journeys to and from its den or burrow. The
final step, the ingestion of the spores, is presumably taken when the animal grooms
itself, as mammals (other than human children) habitually do. Some coprophilous
hyphomycetes (e.g. Graphium) produce slimy droplets of conidia at the top of tall
conidiophores or synnematal conidiomata. These spores are presumably dispersed by
arthropods which may themselves specialize in seeking out dung, and may thus act as
specific, and very efficient, vectors for the slimy-spored fungi.
Many other dung-inhabiting fungi are less specialized than those I have just
mentioned, or have specializations so subtle that we have not yet detected them.
Nevertheless, the fact remains that with patient and repeated examination, we can find a
large number of fungi representing most of the major fungal groups on the dung of many
herbivorous mammals. Repeated observations will show that the various fungi tend to
sporulate in a sequence. First, zygomycetes will appear; then ascomycetes and
conidial fungi, and finally basidiomycetes.
So we can assume that an assortment of spores of coprophilous fungi will be present in
dung when it is deposited, and that these will all have been triggered to germinate by
some aspect of passage through the mammalian gut. While Pilobolus is producing
its miniature artillery extravaganza, the other fungi are growing and assimilating
steadily within the dung, preparing for their own appearance at the surface. The new
hypothesis had neglected only one important factor: antagonism. After a few
weeks, almost the only fungi still sporulating on the dung will be species of Coprinus.
These can go on producing a sequence of ephemeral basidiomata for months. We now know that
the various components of the substrate are far from exhausted after the initial flushes
of growth and sporulation. What has really happened is that Coprinus has seized
control by suppressing most of the other fungi. Hyphae of Coprinus are actually
extremely antagonistic to those of many other coprophilous fungi. If a Coprinus
hypha touches one belonging to Ascobolus, the Ascobolus hypha collapses
within minutes. We don't understand exactly how this trick is done, but it is extremely
effective, and turns out to be a fairly common stratagem among the fungi, whose main
competitors for many substrates are other fungi
Another interesting and important gambit used by Coprinus involves repeated
anastomoses. Spores are more or less evenly dispersed throughout the dung when it is
deposited, and they all germinate more or less simultaneously, producing small mycelia
within the dung. When compatible mycelia meet, they will anastomose, and soon the entire
dung deposit is permeated by what is now essentially a single mycelium, which can then
pool its resources and produce more and larger basidiomata. Cooperation pays off for Coprinus.

There are also some interesting subplots that run concurrently with the main story.
Several of the zygomycetes that usually appear (e.g. Piptocephalis) are actually
parasitic on other zygomycetes. One common zygomycete, Rhopalomyces elegans,
parasitizes nematode eggs. Nematode-trapping fungi such as Arthrobotrys often
sporulate, and develop their characteristic rings and nets (see Chapter
15). Keratinolytic
hyphomycetes such as Microsporum (below) may appear on hair that the animal has
accidentally eaten during grooming.

Occasionally, an undescribed species of fungus may be seen. For many years the third
year mycology class at Waterloo followed the dung succession as a laboratory exercise.
These undergraduates saw the zygomycete Stylopage anomala on horse dung several
years before it was formally described in 1983. They also found an undescribed species of Podospora
(Ascomycetes), which is perhaps the 102nd species of this genus. They also found the rare
zygomycete, Helicocephalum, which I had never seen before.
Horse dung is easy to obtain in most areas, comes in discrete units, and can be handled
and observed without creating much personal distress. As many as 40 species of fungi
representing most major groups of eumycotan fungi are commonly recorded from a single
collection of horse dung. Most of them can be identified fairly easily with the help of
the specialized taxonomic literature that is now readily available, though I admit that
some of the zygomycetes are not easily recognized as such by beginners. Most of them could
be identified to genus with the help of the illustrations on this CD-ROM. Many of
the fungi can be isolated in pure culture without too much difficulty, and with a little
imagination, interesting experiments can be devised to investigate various aspects of
their behaviour. Perhaps now you can understand why I and many other teaching mycologists
ask our classes to put their culturally determined attitudes on hold, adopt an objective
scientific approach, and study the succession of fungi on horse dung, then think about the
biological mechanisms and manoeuvring that lie behind the visible manifestations. It's a
truly thought-provoking mycological experience.
Before we leave this topic I should warn you that you should not collect
and examine the dung of carnivores, because it might support fungi that
could also grow on you. However, in the interests of science, your
humble scribe checked out the dog do-do shown below, which had taken on
a transmogrified appearance in cool, humid weather. I found that the
principal fungus fruiting here is a species of Mucor (Zygomycetes).
I was probably quite safe, because the animal was eating kibble, not cats. But my advice is still the same: stick to herbivores...their droppings don't smell nearly so bad...

If you want to know more about the coprophilous fungi, I recommend
that you read a recent paper: Richardson, M.J. (2001) Diversity and
occurrence of coprophilous fungi. Mycol. Res. 105:
387-402.
I cannot close this section without mentioning a magnificent new book from an Italian physician, Dr. Francesco Doveri (see references). It has 1104 pages, 158 colour photographs and 300 other illustrations. It is undoubtedly the most extensive coverage yet afforded to coprophilous fungi, and took the author 15 years to bring this project to fruition. Definitely worth a look, if you can find a copy.
Now on to a very different habitat...
Amphibious Fungi in Streams
The second area of fungal ecology I want to examine is a stream flowing through a
woodland, somewhere in the temperate zone. We already know that the tiny chytrids and
oomycetes live here, but we might not expect to find many of the typically terrestrial
dikaryan fungi. However, if you collect some stream foam and examine it under the
microscope, you will see that the bubbles have trapped a rather unusual kind of
spore (this is simply a physical phenomenon -- a surface tension effect --
and there is no other relationship between the bubbles and the spores).

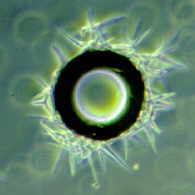
I collected foam in
winter from this stream near where I
live...
If you pass a litre of
this stream water through a filter, then stain the filter in cotton blue
and examine it through the microscope, you will see many large and strikingly
shaped fungal spores. Many, perhaps most, will be tetraradiate.
These two sets of drawings are from
a booklet published by Ingold (who discovered these strange fungi) in
1975.

You can see tha there is a wide
range of different morphologies, almost all of which share one feature -
they have arms or appendages sticking out in various directions.
We will see how these evolved...

Dark field picture of Lemonniera
conidia

Phase contrast photomicrograph of a
conidium of
Lemonniera aquatica

Clavariopsis aquatica
(phase contrast)

Tetracladium marchalianum
(interference contrast)

Articulospora tetracladia
(phase contrast)

Culicidospora gravida (phase contrast)

Others will be unbranched, long, thin
and arc-shaped, sinuate or sigmoid (s-shaped).
Anguillospora, a common sigmoid form
(phase contrast)

They are all produced by conidial
anamorphs that are specially adapted for living in streams.
There are even yeasts with a tetraradiate arrangement of their
cells, presumably for the same reason this shape has been adopted by the
other spores. This photomicrograph is of Candida aquatica.

Where do these spores come from, and how do the fungi that produce them make a
living? The first clue came when limnologists (biologists specializing in freshwater
systems) began to examine the energy budgets of streams. Because some streams flow through
forests, they are heavily shaded during the growing season. This means that few green
plants (primary producers) can grow in them. It was found that more than half, and
sometimes nearly all, of the energy supporting organisms that live in streams comes from
autumn-shed leaves. This source of energy is described as 'allochthonous' (which means
'coming from somewhere else' just in case you wanted to know).
When they first fall into the water, these leaves are extremely unpalatable to stream
invertebrates, but as they are colonized and 'conditioned' by microorganisms, they
apparently become tastier. Experiments in which batches of leaves were treated with
either antifungal or antibacterial antibiotics showed that the fungi were chiefly
instrumental in making leaves palatable to animals such as Gammarus pseudolimnaeus,
a numerous amphipod crustacean living in the stream (another amphipod lives on
the beach below my house in
millions, eating decaying tidal jetsam, mostly seaweeds and, no doubt, the fungi growing on and in
them).
Gammarus, a detritivorous and mycophagous amphipod crustacean

In a feeding experiment, Gammarus (the dark,
comma-like objects) choose to eat fungal
mycelium (the greyish stuff at lower right) rather than unconditioned leaf
discs (dark circles).

Later experiments with leaves conditioned by individual stream fungi showed that not
only were some of the fungi that produce tetraradiate or sigmoid conidia most active in
conditioning leaves, but their mycelia and sporulating structures were also highly
nutritious food for detritivorous stream animals such as Gammarus
(Amphipoda, Crustacea). An important
ecological role had been established for these fungi.
But many questions remained. Were those fungi with tetraradiate spores related to one
another? Did they have teleomorphs? (which would help to answer the first question). Since
streams always flow the same way, and have a natural tendency to carry small things like
spores downstream, where did the inoculum for the upper reaches come from? What were the
advantages of the tetraradiate and sigmoid spore shapes? The information we needed was
gradually accumulated over several years of experiments, until eventually we were in a
position to give some answers.
Many of the tetraradiate (4-armed) spores, though similar in configuration
at maturity, developed in rather different ways. I will describe just two
of these. In some, three arms grew upward and outward from the top of the
first-formed arm. In others, one arm grew upward, the other three or four
outward and downward at the same time from a central cell. Some of these
conidia were thallic, some blastic. A few had clamp connections,
like Taeniospora gracilis, shown here, and were clearly basidiomycetous...
But most
didn't. This impression of diversity was confirmed when some of the teleomorphs were
discovered. Some were unitunicate ascomycetes, both operculate and inoperculate, producing
apothecial and perithecial ascomata. Some were bitunicate ascomycetes. Some were
basidiomycetes.
It became clear that the morphologically similar anamorphs were
actually a mixed bunch: fungi of very different origins that had undergone convergent
evolution, molded by selection pressure into similar shapes. The teleomorphs also provided
one answer to the question of how these fungi got upstream: ascomata and basidiomata,
unlike the anamorphs, were not submerged in streams, and they liberated airborne
ascospores or basidiospores. The group has been christened the amphibious fungi,
because of its immersed anamorphs and emergent teleomorphs.
But why did so many of these taxonomically diverse amphibious fungi evolve conidia with
similar shapes? It was found that as they were carried along by the water, tetraradiate
spores sometimes entered the layer of still water just above the surface of
submerged leaves, and then made three-point landings
on these leaves. We know that a tripod is the most stable configuration,
able to stand firm on irregular surfaces. The spores formed microscopic
tripods that gave them a foothold on the dead leaves for long enough to
germinate from the ends of the three arms, and attach themselves to the
substrate before being swept away.
Much of the
early work on stream fungi was done by Terence Ingold, who published many
papers on the strange fungi to be found on leaves in water, starting as
long ago as 1943.
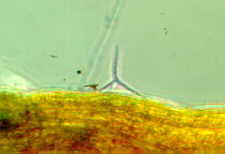
The reason for the sigmoid shape has not yet been fully
established, but Webster and Davey (1984) published a paper: 'Sigmoid
conidial shape in aquatic fungi' in Transactions of the British
Mycological Society 83: 43-52. They observed that most such conidia
tended to roll along in flowing water with their long axes at right angles
to the current, though some vaulted end over end when they touched a
surface. Both kinds of movement bring the ends of the spore into
contact with the substrate. If the flow slows down (and it is almost
zero at the boundary layer next to the substrate), this gives the end of
the conidium a chance to stick to the surface. After attachment, the
conidia swing around and lie with their long axis parallel to the current,
and rarely become detached again. They then produce sticky appressoria,
and germinate quite quickly, apparently stimulated by the
contact.
Sigmoid conidia may represent an evolutionary compromise. Although not as efficient at attachment as tetraradiate spores, they represent a more efficient allocation of resources. As usual in the living world, there is more than one answer to a particular problem
After
colonizing the leaves, the amphibious fungi sporulate again, and it was found that they
would do this only in highly oxygenated conditions, and with the physical stimulus
provided by flowing water. It is clear that amphibious fungi
incorporate many special adaptations, both morphological and
physiological, to their environment.

If the spore numbers are charted over the entire year, it will be seen that their
numbers peak in Fall and Spring. In the first place, the massive new input of autumn-shed
leaves provides the necessary substrate. In the second case, spring run-off will also
carry plant debris into the stream. The entire process is diagrammatically summarized
below, showing that the fungi are vital intermediaries of energy flow in streams,
providing a link between dead leaves and trout.

Nikolcheva et al. (2005) have used molecular
techniques to explore diversity of fungi in the early stages of
colonization of leaves in streams (see references), and showed that after
initially high diversity, numbers of taxa fell as terrestrial fungi were
outcompeted by aquatic species, and aquatic species established their own
pecking order.
Aero-Aquatic Fungi in Ponds
One good aquatic habitat deserves another, so after sorting out the role of fungi in
streams, we switched our attention to woodland ponds (our third habitat).
The pond in question lay in the heart of the woods behind my house in
Waterloo.

Again, primary production within the pond was limited by the forest canopy. Again,
there was a specialized group of fungi living in the pond, though no-one knew if these
fungi played an important role in the ecology of the pond. In this case the fungal
propagules commonly found were hollow, and floated. Again, this end was achieved in
several different ways, of which I will describe only two. The pond has almost dried out
in summer (1) A conidiophore emerges from a dead leaf just below the surface of the the
water, and branches like a tree. Eventually, the ends of the fine branches all swell up
and fuse with their neighbours to form an air-filled, watertight structure. This is the
propagule of Beverwykella.
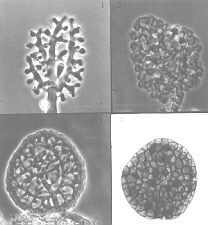

(2) Another conidiophore grows from a dead leaf, emerges through the water surface,
and its tip begins to grow in circles. Coiling repeatedly on itself in wider and wider,
then narrower and narrower gyres, it eventually builds a barrel-shaped, air-filled,
watertight structure. This is the propagule of Helicoon.

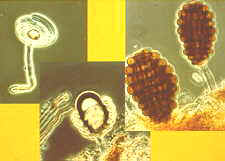
The fruit bodies (magnified X 10 in the top left picture, and X 50 in the top right picture)
are hollow, and are lined with non-shooting basidia (bottom right X
1500). Note the symmetrical mounting of the spores and the lack of a pointed
sterigma (see discussion in Chapter 5b).
A young basidium (bottom left) shows the typical clamp connection at its base.


Here is another apparently rare pond fungus, the tiny floating
gasteromycete, Limnoperdon. It has been recorded only from our pond in
Ontario and somewhere near Seattle, Washington, though it surely occurs at many places
between those widely separated localities -- people just haven't looked carefully.

Because these fungi live and grow under water, but produce their spores only above the
surface, they are called the aero-aquatic fungi. It's obvious that the
structures of the two kinds of conidia described above, though functionally
equivalent, are not closely related. Again, convergent evolution has been at work, the
selection pressure applied by some ecological imperative.
We finally discovered what this was. It was the need to be first on the scene when
new substrate appears. When a dead leaf falls into a pond, it does not sink immediately.

It may actually fall on top of some of the floating propagules just illustrated, or
the propagules may be drawn to the floating leaves by surface tension. In either case,
these fungi will be the first pond-adapted species to enter this new substrate. The leaves
soon sink to the bottom of the pond, carrying their new colonizers - hyphomycete or
gasteromycete - with them

These fungi also have the ability to grow at low oxygen levels, and to survive the
virtually anaerobic conditions that prevail at the bottom of a pond for extended periods
during the winter.

Sporulation will happen again when the pond begins to dry out during the following
summer, and the water level subsides until the colonized leaves are once more just below
the surface. We found that these aero-aquatic hyphomycetes play an ecological role
parallel to that of the amphibious fungi in streams: conditioning the dead leaves, and
making them palatable to the detritus-eating invertebrates such as snails, and vertebrates
such as frogs, whose larval stages live in the pond.
Frog spawn [below]

Produces tadpoles which skeletonize leaves
after the fungi have 'conditioned' them...

...and eventually metamorphose into
tree frogs [bottom] which represent the apex of the pyramid of life in the pond.

There are quite a few marine fungi, including
many ascomycetes, some hyphomycetes and a small number of basidiomycetes,
but all the evidence, both morphological and molecular, points to
terrestrial origins for these fungi.
Other Habitats
The biosphere has myriads of other habitats, each unique in various ways, and each making
special demands of the organisms that live in it. The roots of plants create special
conditions around themselves, and have established especially intimate relations with
hundreds of endotrophic and thousands of ectotrophic mycorrhizal fungi (which have Chapter 17 to themselves). Other rather less
specialized saprobic and parasitic fungi also abound on and near roots. The surface of
living leaves is inhabited by a specialized mycota, while dead and decaying leaves are
substrates for a succession of other species. The soil, into which most leaf remains are
incorporated, is itself a mass of microhabitats, and is the richest reservoir of fungal
diversity. And of course the leaves of different plants, and the various soil types, will
have different subsets of the total mycota. Juliet Frankland, in her
Presidential address to the British Mycological Society (reference below), gives a nice
overview of the problems and progress in our study of fungal succession, exemplifying them
with an autecological study of the agaric, Mycena galopus.
Not all fungi can be
parceled out neatly into successive steps of a succession. Often, fungi compete for
access to a substrate. Sometimes a natural phenomenon will give us an unexpected insight
into this struggle. Here is a picture of a block of wood which has been colonized by many
different mycelia. The boundaries between the 'territories' of different mycelia can be
clearly seen as black lines or zones, and the wood is described as 'spalted.'
The black material is melanin-like, oxidized and
polymerized phenolics deposited by wood-rotting fungi, and although the biological
function of the zones isn't entirely clear, melanins are the precursors of the humic
acids, which are long-lived and important determinants of soil fertility.

This kind of partition can even occur in much smaller substrates, such as individual
leaves, as in this one of Oregon grape (Mahonia) from John
Dean Provincial Park near where I live. The colonies and black fruit bodies shown are of Coccomyces
(Rhytismataceae, Ascomycetes). The lower pictures
are
close-ups, which show the unusual
polygonal outline and stellate opening
of the ascomata. Note that while the colonies in the
second picture have produced only one ascoma each, that in the third picture
has a larger territory, and has been able to develop multiple ascomata, now
open to expose the hymenium.



Another fungus exploiting leaves of
Mahonia is Cumminsiella (Pucciniaceae, Teliomycetes).
This fungus attacks living leaves, and thus preempts the saprobic
Coccomyces.

This time a single infection
occupies a whole leaf. The uredinial sori open on the lower surface of the
leaf.
There's more information on this fungus, including a photomicrograph of
urediniospores and a teliospore, in Chapter 5d.

This senescent salal (Gaultheria shallom) leaf has also
become divided up, but in this case two or three different fungi are involved.



Even if a leaf isn't subdivided into territories, after it dies you are
likely to see fungal colonies develop, as is happening in the Hosta
leaf (above, left) from our garden. The fungi in this case are mainly Cladosporium
and Epicoccum, two common saprobic hyphomycetes. The picture
on the right shows a part of another Hosta leaf, clearly
demonstrating that the areas covered by the fungal colonies, marked X, are
the first to be eaten by animals. We may compare the fungi to peanut
butter, and the leaf to the bread which it renders palatable.
Which brings me to the subject of my own PhD thesis -- the succession of fungi
involved in the long, slow decomposition of another kind of leaves -- Scots pine needles
(our fourth habitat).
I was presented with a problem which, briefly stated, was as follows. "When we isolate fungi from the soil, the majority of cultures will be of light-coloured fungi, while a majority of the hyphae seen in the soil are darkly pigmented. Figure out what's going on."
I chose to work in a pure stand of Scots pine (Pinus
sylvestris)

I looked at the various soil horizons
in the forest, and tried innumerable times to grow the dark hyphae, picking them out with a
micromanipulator and giving them a variety of delicious media. But they refised to
grow, so I eventually decided that most of them must be dead, and that they had perhaps
grown at some other time and in some other place. I looked in the organic horizon
above the mineral soil, and found there a thriving community of litter decomposing fungi,
which I proceeded to investigate (I did not realize it at the timer, but this is fairly
typical of PhD projects, which are often changed in mid-course by some unforeseen
event(s).
I embedded a small chunk of the Scots pine forest floor in cold-setting
resin, then cut it into vertical slices, one of which I drew for this illustration, which
shows the spatial distribution of needles, and their gradual transition from L >
F1 > F2 > H layers. I then decided to examine as many needles from each layer and
sub-layer as I could process each month (the number turned out to be 300 needles).


The vial (above) contains living needles from the tree, which
represented stage one in fungal colonization. Vial 2 = L layer (pale brown), vial 3
= upper F1(much darker, but still tough), vial 4 = lower F1 (blackish and softer), vial 5
= F2 (greyish and fragmenting). By the time litter material entered the H layer, it was no
longer recognizable as individual needles.
Needles were treated in various ways. (1) Some were washed repeatedly to remove loose
surface spores and plated out in segments to isolate fungi on and in the needles. (2) Some
were surface sterilized before plating out, to select for internal colonizers. (3)
Some were wax embedded and sectioned. (4) Some were observed directly over a period of
incubation in damp chambers.
This is a reference point -- a transverse section of a healthy,
living needle. Changes can be measured against this.

And here is one of the first dramatic changes, the development of numerous
ascomata of Lophodermium pinastri, which apparently often colonizes the interior
of living needles without producing overt symptoms. The death and fall of the needle
stimulates the fungus to fruit.

The large number of lenticular black ascomata of Lophodermium
that can occur in a single needle indicates a dominant colonizer. Here two ascomata are
seen in section.

Other fungi fruit in other needles -- note the several paths along which
individual needles may travel. In this picture, the fruit bodies are pycnidial
conidiomata of a coelomycetous anamorph, Fusicoccum (holomorph probably Botryosphaeria).
The interior of the needle can be seen to be breaking down under the attacks of the
fungus.

Meanwhile, on the surface of the needle, networks of dark hyphae (remember
them?) develop. But what fungi do they represent? Mycelium without sporulating
structures is not very helpful unless one has access to molecular techniques.

Fortunately, several of these fungi fruited either in nature or in the
damp chambers. The first of these is Slimacomyces monospora (which I
mistakenly described as a species of Helicoma in 1958!)

Here is a drawing of Slimacomyces monospora, with its single
helicosporous chlamydospores.

A second major surface colonizer is Sympodiella acicola which I
described as the type species of a new genus (It still stands). Again, note that the conidiophores are in an
almost pure stand.

Here is a drawing of Sympodiella acicola, showing that its unique
characteristics are that while its conidiophore extends sympodially, the conidia are
thallic-arthric (for those of you who are fans of
conidium development -- otherwise look back to Chapter 4).
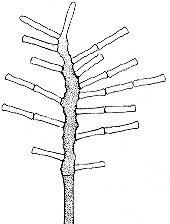
Another fungus that is obviously at least partly internal develops
sclerotia and conidiophores. This was Thysanophora penicillioides...

And here is part of a Thysanophora conidiophore with a
penicillus (the brush-like conidiogenous apparatus - the numerous cells at
the top are phialides, which can each produce many conidia in a dry
chain).

Another pure stand of external conidiophores of an internal fungus, Verticicladium
trifidum (an anamorph connected to an apothecial fungus, Desmazierella acicola,
which I never saw). The conidiogenous cells of this fungus extend
sympodially during conidiogenesis.

Finally, we have arrived at the partitioning of needles among fungi.
It is particularly obvious here, where a darkly pigmented fungus squares off
against an apparently unpigmented fungus.

Section of a partitioned needle, showing the melanin barrier between
species. The fungus at upper left is Verticicladium; its neighbour is not
fruiting so cannot be identified. Compare this partition with the black lines shown
earlier in wood and leaf.

Now a new participant bursts onto the scene (well, it's aleady left, but
it has left its trademark - frass). Now the needles have been softened up by the
fungi, arthropods can eat the needle material. The frass identifies the intruder as
an oribatid mite.
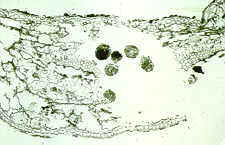
And here it is, a miniature armoured tank that eats fungi and needle.
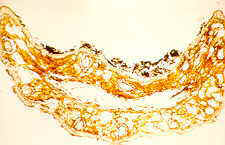
Now in the lower F1, the interior of the needle has collapsed or been
eaten, and the upper surface is coated with a deposit of frass, which contains many
fragments of fungal hyphae and spores.
This diagram plots the overall picture, following the needles through 9
years of mainly fungal decay. The width of each bar represents the relative
importance of the fungus at each stage. Darker bands show fruiting periods. At far left
the fungi are those that grow on or in living needles. As we move to the right, the fungi
involved in later stages of decay are traced.

Read this table carefully -- it will amaze you, and it shows just how
important fungi are in the forest ecosystem. Not merely important, but producing
greater biomass than any group other than the plants.

A recent paper by Paulus et al. (2003 - see references) gives
further insight into what is going on in decaying plant
litter. Using a particle filtration technique, these authors
isolated no fewer than 1365 isolates, representing 112-141 morphotaxa,
from 8 leaves of Neolitsea delabata in the tropical forests of
Queensland.
Fire fungi
Other special substrates have evoked their own suites of specialized fungi: keratin is attacked by some of the Onygenales (Ascomycetes) and their anamorphs; wood by many Aphyllophorales and some Agaricales. Extreme physical conditions (see fire, above) have selected specialist fungi which, by evolving the ability to cope with high or low temperatures, or low water activity, have essentially escaped from competition, and gained access to untapped food supplies. Some fungi are the most osmotolerant organisms known (see Chapter 20). The cycling of anamorph and teleomorph, which I mention many times in connection with plant disease fungi in Chapters 4 and 12, is often largely a matter of their response to specific ecological conditions, which turn on and off large segments of the genome. The fungal ecology of sewage, compost, mushroom beds, agricultural and forest soils, naturally decomposing plant remains, some cheeses, bread, wine and beer, crops in the field and after harvest, the air, the space between your toes, and the tissues of immune-deficient or immune-suppressed people: all can be the subjects of worthwhile, and even important, studies of fungal ecology. Many of the food webs illustrated in ecology textbooks miss out more than half of the organisms involved in the transfer of energy and nutrients. They often stress macroscopic organisms, while omitting microscopic organisms such as the saprobic and mycorrhizal fungi. This neglect is unfortunate, especially since we now appreciate that microorganisms, being at the base of food webs, provide nutrients and mutualistic symbionts for almost all plants and animals. The basic links in terrestrial food webs lie in the soil which is, of course, where a huge number of fungi still live. Every attempt to understand trophic systems must start and finish with soil organisms. And surely the fungi are among the most important of those.
Macrofungal
Ecology - Help wanted!
Most of the situations I have described in this chapter are small or
localized. If we consider the macroscopic fungi, and their roles in
such extensive ecosystems as forests, we find that the state of fungal
ecology is relatively primitive, meaning that we simply don't know very
much about how those fungi act and interact under natural
conditions. If you doubt this, you could explore the mycological
literature for information on where to find morels (in my opinion, the
best of all edible fungi). You will be led a merry dance, from old
apple orchards and dead elms to recently burned forests. Until
relatively recently, no-one even seemed to know whether morels were
mycorrhizal or not (my understanding is that they are opportunistic
saprobes, exploiting new substrates then fading away, only to appear
somewhere else when new food sources present themselves).
As for the ubiquitous agarics, which are undoubtedly the most widely collected and studied
of all fungi, I have to report that things aren't much better. Only Europe holds out a candle in the
darkness. Since europeans have been collecting and recording
macrofungi for centuries, they have the kind of database that allows the
present generation of mycologists to draw comparisons with the past.
This is why several European countries have 'Red Lists' compilations of
macrofungi which seem to have undergone serious declines in recent years
-- or even to have disappeared altogether. It is impossible to
produce such red lists for anywhere in North America because records do
not go back far enough and are, in any case, still fragmentary. Although we may suspect that certain species are
declining or disappearing, we have no well-documented historical reason
for saying so. You can find out more about red lists by googling 'red list
endangered fungi'.
We understand that about half of the known agarics are mycorrhizal -- they
have an intimate, mutually beneficial relationship with many of our forest
trees, and ecological research has recently begun to focus on the effects
on such fungi of various forest practices, and especially the
clear-cutting of old-growth forests which still (regrettably) goes on in
many jurisdictions, and most blatantly in British Columbia where I live.
One of my own graduate students has recently established that many of the
fungi associated with old growth forests do not re-colonize
clear-cut habitats for 40-50 years. And his suspicion is that
the recolonization happens by means of airborne basidiospores which
originate in nearby old growth. What if there is no longer any
nearby old growth to provide this inoculum? But International
logging companies carry on in blissful ignorance of any such
concerns.
Just when we think we have established a few
principles based on the occurrence of fruiting bodies of the mycorrhizal
fungi, it is demonstrated by molecular techniques that in many cases the fungi producing the
fungal sheaths around the roots of the trees are not those whose
basidiomata are appearing above ground. Are we back to square
one? No one seems sure at present. But I mention this to
demonstrate how little we actually know about macrofungal
ecology. Some recent studies (2005)
claim that the apparent disconnect is at least partly because many of the
mycorrhizal fungi develop small, inconspicuous, cryptic or seasonally
limited fruit bodies, rather
than the large and conspicuous ones detected by most collectors.
A fascinating study by Tofts and Orton (1998) points out that although they
have been collecting agarics regularly in a particular woodland in
Scotland for 21 years, and have recorded 502 species in that time, each
year they still find species they have never seen before. Over twenty
years of collecting, and they still cannot say that they have a proper handle on agaric biodiversity in that woodland. They suggest that
at
least 25 to 30 years of collecting, and possibly more, will be necessary
before that goal can be attained.
An even more recent paper by Straatsma, Ayer and Egli (2001) emphasizes
many of the same points. They collected basidiomata weekly for 21
years (1975-1999, except for 1980-1983), and recorded over 400 species in
a 1500 m2 plot. Yet only 8 species were found every
year. The number of species/year ranged from 18 to 194, and even in
the last year of the study, 19 species appeared which had not previously
been found. Clearly, the authors had not seen the full diversity of
macrofungi.
A group of west-coast mycologists (including Paul Kroeger, Christine
Roberts, Oluna and Adolf Ceska, and me), supported by the Mellon
Foundation, has been doing a macrofungal inventory of Clayoquot Sound on
the west coast of Vancouver Island. Over 5 years, visiting once in
spring and twice in fall, we have collected 660 species. Two of the
most interesting features of our study have been: (1) that
only 38 species were found every year and 407 were found only once, and
(2) that a large number of fungi new to the study cropped up
each year. After year one, we have found about 100 additional
species each year.
In Fall 2004 the Cascade Mycological Society held its 16th successive mushroom fair at the Mount Pisgah Arboretum just outside Eugene, Oregon. As a guest speaker for the Society I was fortunate enough to be invited to participate in the collecting trips leading up to the fair. The fair is an exciting introduction to the larger fungi because over 300 species are usually on display - speaking to a huge effort on the part of the members. I was also fortunate enough to get my hands on the statistics for all sixteen years. Over those years just over 700 species have been recorded from the area. When we arranged the data according to the number of years in which each species had been collected, an interesting picture emerged.
To begin with the extremes. I was rather surprised to discover that only 37 species (just over 5% of the total number) had been found in all sixteen years. It was equally thought-provoking to learn that no fewer than 190 species (almost a third of the total) had been recorded only once in those 16 years. Here is the list of species versus years.
Species No.
of years recorded
37
16
44
15
31
14
24
13
20
12
13
11
18
10
18
9
22
8
22
7
22
6
41
5
29
4
48
3
92
2
190
1
There are possible flaws in the data set. For example, species may have been misidentified. But the general trend is obvious. A relatively small number of taxa will show up every year, or almost every year, while a much larger number of taxa will be found much less often, and a very large number will be encountered only once every decade or so.
How many more taxa will show up in the years to come? What is the full number of species that the Cascade group can expect to find if they keep at it long enough? If we may be allowed to take a quick look in the crystal ball, might we not find that after 50 years they will have found 1,000 species?
This data set (for which I am indebted to the hardworking collectors and record-keepers of the Cascade Mycological Society) points up the necessity for very long-term studies wherever the diversity of fungi is to be fully explored, and calls for the accumulation of much concurrent data on weather conditions and other ecological factors if we are to understand why some fungi are so notably shy.
These reports are not intended to put you off, to deter you from getting involved in fungal ecology. Rather the reverse. It is clear that the need for research in this area is critical. We need good ecological studies just as much as we need molecular research on fungi. Some groundwork has been done in Britain, where the macrofungal assemblages characteristic of many habitats have been broadly outlined. But this is still far from an understanding of the full role played by those fungi in the habitats being considered. The need for seminal research has never been greater. The next section discusses some of that.Fairy Rings
Fairy rings are one of the few fungal phenomena that most people have seen. To the superstitious mind the arrangement of the fruit bodies in a circle might seem very strange. On top of that, the grass often doesn’t grow just inside the fruiting zone, so it looks really weird, and it isn’t too surprising that in the past, people ascribed such rings to supernatural events (fairies or witches dancing).
The real explanation is simple enough, once you know that mushrooms start life as microscopic spores that germinate, develop branching hyphae, and soon grow outward from their point of origin as a mycelium that tends to spread equally in all directions (see Chapter 3a), and thus forms a colony that describes an ever-widening circle in the soil. When the mycelium has accumulated enough food, and conditions are right, mushrooms develop at the periphery of the circle, finally bringing the previously invisible colony to our attention.
Fairy rings become larger every year, as the mycelium grows outward, always looking for food. It can’t turn back because it has used up the food resources behind it. Growth of the grass is inhibited because the dense fungal mycelium prevents water from penetrating the soil, and possibly because the fungus has released metabolites inimical to the plants. The grass inside the rings sometimes grows more lush, because the fungus has liberated nitrogen by its activities. Some rings, on places such as Salisbury Plain in England, that have been under grass for many centuries, are estimated to be at least 400 years old. It is quite possible that much larger and therefore even older ones could be found. You might keep your eyes open.
Many species of agarics (perhaps as many as 60) produce fairy rings. The most obvious are those which occur in pasture, and are often generated by species of Agaricus (see Chapter 5b), or by Marasmius oreades, which is actually called the 'fairy ring mushroom' (see photograph below). Sometimes another kind of fairy ring develops around trees, as mycorrhizal fungi grow outward from the roots.

The Humungous Fungus
Some years ago, mycologists were working on a forest in Michigan where three
species of Armillaria, well known pathogens, had been causing
trouble. They found that one of these species, Armillaria gallica,
seemed to be monopolizing a large area of the forest - 15 hectares.
When molecular biologists checked samples from within this area, they
found that all belonged to a single genet (a product of sexual
reproduction). So here was a single species of mushroom that had
spread through the soil and covered an amazing 15 hectares (35
acres). How was this achieved?
Armillaria
gallica apparently had a secret weapon. It soon became apparent
that this was its rhizomorphs, well-organized hyphal strands with
conducting hyphae in the centre, and a dark protective rind on the
outside, which help this fungus spread through the soil protected from the
hostile influences so common in this medium. Armillaria gallica
sends out rhizomorphs through the soil in search of new food
substrates. It isn't a particularly pathogenic species, so its
rhizomorphs wait patiently until a tree is weakened or dead before
invading. Having established its new base, it sends out more
rhizomorphs...
Sampling
established that there were about 10 tonnes of rhizomorphs in the
genet. If the fine assimilative hyphae and other structures were
factored in, the total mass of the colony/genet added up to 100 tonnes -
about the size of a blue whale. It was also calculated that the
colony was at least 1500 years old.
No sooner had the credentials of this fungus been established than a larger genet was discovered in Washington State. This one belonged
to Armillaria ostoyae, the common west coast species of Armillaria, and it
covered an almost incredible 600 hectares (1500 acres). Even more
recently, an even larger genet of Armillaria ostoyae has been
identified in the Blue Mountains of north-eastern Oregon: this one covers
2200 acres (about 900 hectares) and is estimated to be
between 2,400 and 8,500 years
old. This is
among the largest and most extensive organisms on earth. Who said
fungi were insignificant?
Diploidization-Haploidization and
Genetic
mosaics in Armillaria
It is possible that one of the reasons for the long-lasting success of
these species is a rather radical departure from the genetic norm.
It has recently been discovered that in Armillaria gallica and
A. tabescens, the
individual nuclei in the cells of the mycelia and of the non-basidial
parts of the species are haploid (not dikaryotic). This shows that
before the mushroom developed, there must have been events similar to
those that usually occur only in the basidia - the fusion of two
compatible nuclei and a subsequent reduction division: an extra-basidial
diploidization-haploidization event. It is obvious that for each
such event, new genetic variation is introduced to the organism
(crossing-over during meiosis ensures this) So mushrooms of at least
two Armillaria species are apparently mosaics of genetically distinct
nuclei. When these nuclei are incorporated into basidia, and undergo yet
another diploidization-haploidization event, even more diversity is
introduced to the organism. Since species of Armillaria
represent some of the largest and oldest organisms on Earth, it seems
possible that this 'mosaicism' may confer additional genetic flexibility
on these organisms, thus contributing to their amazing success. This
development of genetic mosaics has thus far been discovered in only two
species of Armillaria, but it is apparent that we are at the
beginning of another area of genetic exploration and discovery in the
mushrooms (see Peabody et al. refs below).
An article by Tom Volk, which can also be found here
is reproduced below. It is
written in an accessible style, and has some good pictures -- I'm pretty
sure you will enjoy reading it!
The Humongous Fungus--Ten Years Later
Thomas J. Volk, Department of
Biology, University of Wisconsin- La Crosse, La Crosse, WI 54601
volk.thom@uwlax.edu

Has it already been ten years? On April 2, 1992, the non-mycological world
first became aware of very large fungi, thanks to the efforts of Myron
Smith, Johann Bruhn, and Jim Anderson. They published a landmark
article in Nature (Smith , M., J. Bruhn and J. Anderson, 1992. The
fungus Armillaria bulbosa is among the largest and oldest living
organisms. Nature 356:428-431), but no one expected the media blitz
and the scientific interest that would follow.
First you'll need to know a bit of background on Armillaria, also known as the Honey Mushroom. Armillaria (Fr.:Fr.) Staude is a genus of mostly pathogenic agaric fungi. Perhaps the most important aspect of the life cycle of Armillaria is the formation of rhizomorphs, conglomerations of differentiated parallel hyphae with a protective melanized black rind on the outside. The rhizomorphs are able to transport food and other materials long distances, thus allowing the fungus to grow through nutrient poor areas located between large food sources such as stumps. The rhizomorphs can also act as "scouts" for the rest of the thallus, searching for new food sources. These proliferative rhizomorphs apparently permit Armillaria colonies to spread and become quite large. Thus enters the Humongous Fungus.
The science that led to this seminal Nature publication (Smith et al. 1992) turns out to be quite interesting and very thorough. The project was actually an offshoot of a grant from the Department of Defense, which funded a project to study the possible biological effects of ELF (Extra Low Frequency) stations in the Upper Peninsula of Michigan. These ELF stations were built to communicate underground with ocean-going submarines in time of war. The humongous fungus site, which Johann Bruhn had been studying for many years, was actually one of the control sites, nowhere near the ELF stations. Historically, the site (near Crystal Falls, Michigan near the Wisconsin border) had been mostly northern red oak/white birch/ sugar maple forest, but the native trees had been harvested with more profitable red pines planted in their place. When the oaks were cut, the stumps were mostly left in the ground to rot. The oaks had been infected with Armillaria root rot, but had survived very well because they were not under any stress. However, when pines were planted, some species of Armillaria were able to kill the young pine seedlings. The particular species that garnered their attention was Armillaria bulbosa, which is now correctly known as Armillaria gallica. I'll tell you more about this taxonomic problem later.
The study of Smith et al. (1992) was performed by collecting vegetative mycelium of Armillaria by "baiting" with small pieces of poplar wood, actually popsicle stick-like tongue depressors. Since Armillaria is a wood decay fungus, the mycelium quickly colonized the tongue depressors. The labeled inoculum stick could then be easily collected. Additional subcultures, including tissues and single spore isolates, were made directly from fruiting bodies that appeared in the fall or from the black rhizomorphs and mycelial fans that are always present in the soil or on the wood, especially under the bark. The laborious process of analysis began, first with checking the mating type loci by mating on media in Petri dishes. Molecular techniques were then employed, first looking at mitochondrial DNA (mDNA) restriction patterns. These were both good markers for the study because mating type loci and mDNA restriction patterns are both highly variable within Armillaria species. Once these were determined, RAPD (Random Amplified Polymorphic DNA) and RFLP (Restriction Fragment Length Polymorphisms) markers were employed to check for additional heterozygous loci in the nuclear genome. With these several types of data, they could begin to draw maps of the area to determine the limits of each individual. One of these clones turned out to be quite large, covering 15 hectares (37 acres). Within this area, all the vegetative isolates had the same mating type , the same mDNA restriction pattern, and had the same eleven RAPD products and five RFLP-based markers, each marking a heterozygous locus. These data indicated that this 15 hectare clone is a single organism. However, some argued that this only meant that they had the same alleles at these genetic loci, bringing up the possibility that the samples were from separate, but closely related organisms (or individuals) that arose from separate matings. In their paper, Smith et al. (1992) presented some complex statistics that show that the probability of this being the case was infinitesimally small (P= 0.0013), given that all the samples share all the heterozygous markers examined. Even so, to lend even more credence to their conclusions, eventually they tested 20 RAPD and 27 nuclear restriction fragments that were found to be invariable in the large clone. By far the most likely hypothesis is that this clone reached it enormous size through vegetative growth. Thus the Armillaria clone was proven to be quite a humongous fungus. By estimating (very conservatively, I might add) the growth rate of the fungus under their natural conditions and by extrapolating the weight of the clone from smaller soil samples (again very conservatively), Smith et al. found the clone to be at least 1500 years old and weigh at least 9,700 kg (more than 21,000 pounds or 100 tons), close to the mass of an adult blue whale. They compared the mass to that of a giant redwood (Sequoiadendron giganteum) estimated to be about 1,000 tons, most of which is dead xylem tissue. The conclusion of their paper states, "This is the first report estimating the minimum size, mass, and age of an unambiguously defined fungal individual. Although the number of observations for plants and animals is much greater, members of the fungal kingdom should now be recognized as among the oldest and largest organisms on earth."
Myron Smith wrote to me recently in an email "I have often been asked something like,'what made you look for a large fungus?' Like most discoveries (and this is a point that needs to be stressed to granting agencies), we did not set out to make this discovery. Initially, (at least when Jim and I came on the scene) we wanted to find out how mitochondrial DNA was inherited in fungi in nature (Smith, Duchesne, Bruhn and Anderson, 1990). The first year we went out and sampled from a 120 x 60 m area. Nearly every sample was identical for mDNA and mating type. The second year we extended our sampling over a 1 km transect through the area and, again, detected this one wide-spread genotype. By extending the areas sampled in subsequent years, we were finally able to delimit the large area occupied by this genotype and then go on to show that this genotype likely represents an 'individual'."

Although they knew they were publishing a very good paper, Smith, Bruhn, and Anderson never expected what happened next. On that historic publication day, the furor began. Johann Bruhn, at that time at Michigan Technological University in Houghton, Michigan, now at the University of Missouri- Columbia, received the first of many phone calls from the media. Since it was April 2, he thought that this was a late April Fool's joke, but soon more calls began pouring in. All of the major television networks called; all of the major newspapers called from around the world. CNN called and reported that they had a plane in the air and would Johann please drive over to the site and wave so that they could take photos of the fungus. One Japanese businessman called and wanted to set up a partnership to build a boardwalk around the humongous fungus and charge people to view "the pulsating mass of fungus" that was there. Johann reports shutting himself into his office and having the secretary screen the calls one at a time as they came in. The two authors at the University of Toronto, Myron Smith (now at Carleton University in Ottawa) and Jim Anderson (still at the University of Toronto) experienced a similar media deluge. I first became aware of the media hype as I heard Jim Anderson being interviewed on US National Public Radio. You'll have to talk to the three authors to hear further interesting stories.
The media blitz lasted a month or so, then seemed to dissipate as things got back to normal. However, on May 18, 1992, it all began again. Terry Shaw, then in Colorado with the US Forest Service, and Ken Russell, of the Washington DNR, reported that they had been working on an even larger fungus, Armillaria ostoyae, that covered over 600 hectares (1500 acres, 2.5 square miles) south of Mt. Adams in southwestern Washington. The newspaper headlines read "Humongous Fungus has BIG brother out west." The fungus wars had begun. Who had the larger fungus? Questions arose as to who had better proof that theirs was a single organism. Russell and Shaw had only shown that the mating type loci were the same, but they had beautiful aerial photos showing growth of the large colony in a radial pattern, showing where it had killed the conifer trees. Smith et al. had a much more convincing argument, with several meticulous lines of genetic evidence showing without a doubt that theirs was a single clone.
Ten years later, we are still experiencing the fungus wars. In August of 2000, Catherine Parks of the US Forest Service in Oregon (along with collaborators Brennan Ferguson, Oregon State University; Tina Dreisbach, PNW Research Station, Forest Service; Greg Filip, Oregon State University; and Craig Schmitt, Wallowa-Whitman National Forest, Forest Service) reported that they had found an even larger fungus (again Armillaria ostoyae) in the Blue Mountains/ Malheur National Forest in Eastern Oregon. Their fungus is nearly 900 hectares (2,200 acres or 3.4 square miles or "as large as 1,665 football fields") and is estimated to be more than 2,400 years old. They used methods similar to those of Smith et al., including mating type analysis, but with the addition of DNA fingerprinting, not widely available in 1992. It seems likely that there are larger Armillaria clones out there somewhere. Myron Smith wrote to me: "As far as I (and I think this is true for Johann and Jim as well) was concerned, the 'Fungus wars' were a non issue; another example of sensationalistic journalism. The chance of finding "the largest fungus" is incredibly small. Our main point was how to unambiguously identify a genetic individual. That we did find a large individual by chance, however, suggested to us that massive, old fungi are probably not uncommon."
One interesting offshoot of these findings of humongous fungi has been a scientific discussion of "what exactly is an organism?" Most people understand the concept of an organism in an animal, which has very carefully defined limits--and most of it is usually visible as it moves around. However, much of a typical plant and most of a typical fungus is not visible to the naked eye. In particular with fungi, the limits of the individual are not clearly defined. The large question was "are these humongous fungi acting as single organisms?" It was well proven that the genetics of various parts of the humongous fungus organism are identical, but can, for example, one part of the organism communicate with other parts of the organism? Do they share physiology? If different parts are growing through different substrates, are they supplying other parts of the fungus with missing nutrients? Several articles began to appear in the scientific literature including Gould (1992) in which he spent a great deal of time discussing populations of asexually reproducing aphids. One letter to the editor by James Bullock of Oxford University (1992) pointed out some larger clones of plants, including an aspen clone (Populus tremuloides) covering 81 hectares and over 10,000 years old. At that time Bullock did not know about the larger A. ostoyae clones.
Despite the large size of the mycelia of these humongous fungi, the fruiting bodies (mushrooms) are really quite average in size. However, during a good fruiting season, the honey mushrooms may be quite abundant, producing a widespread biomass. However, the largest single fruiting bodies are produced by perennial polypores (shelf fungi), such as Bridgeoporus nobilissimus, Rigidoporus ulmarius, and even Ganoderma applanatum. Some of these large fruiting bodies may weigh over 160 kg or 300 pounds! Certainly these are much larger than Armillaria fruiting bodies, which are typically 50-100 g each.

I promised to tell you something about why Armillaria gallica is the name we should use for this species rather than A. bulbosa. Armillaria species typically produce a white spore print and have attached to decurrent gills. Most species have an annulus. Delimiting species in fungi is often difficult, but in Armillaria the biological species concept, based on mating compatibility, has gained wide acceptance. Until the late 1970's Armillaria mellea (Vahl:Fr.) Kummer was considered by most researchers to be a pleiomorphic (highly variable) species with a wide host range and distribution. The pathology literature on A. mellea was extremely confusing. The fungus was considered by different researchers to be either a virulent pathogen, an opportunistic pathogen, or an innocuous saprobe. Its host range was one of the broadest known for fungi. It was clear that more than one species must be involved. Because of the difficulty with studying the basidiomata using traditional characters, other methods of study were devised. Hintikka (1973) developed a technique that allowed determination of mating (incompatibility) types in Armillaria based on culture morphology of single-spored (haploid) pairings. He and his colleagues found six biological species in Europe. The work was extended into North America, where Anderson and Ullrich (1979) demonstrated that what had been considered as Armillaria mellea in North America was actually 10 genetically isolated biological species (North American Biological Species or NABS). Anderson, Korhonen, and Ullrich (1983) found that most of the biological species of Europe (including A. gallica, NABS VII or EBS E) were also represented in North America, although the reverse was not true.
There is a bit of controversy about what to call this species. Very briefly, the name A. bulbosa Velenovský (1927) [a.k.a. A. bulbosa (Barla) Velenovský, but Barla's (1887) name A. mellea var. bulbosa was illegitimate] has a very poorly preserved type specimen. Vladimir Antonín (1986, 1990) of the Czech Republic has examined Velenovský's type specimens (preserved in a liquid fixative) and has concluded that the specimen could be any of three species. According to Marxmüller (1992) Velenovský's species is identical with A. cepistipes Velen. (1920), which has priority, being an older name (see also Termorshuizen and Arnolds, 1987). Another name proposed for this species has been Armillaria lutea Gillet, but this species lacks a type specimen, and Gillet's (1874) description could represent any one of three species. Armillaria gallica Marxmüller & Romagnesi (1987), which has an excellent type specimens and abundant cultures, is the only name that can unequivocally be assigned to European Biological Species E and NABS VII. See Volk & Burdsall (1995) for a clear explanation of this taxonomic problem.

No matter what we call the species, the people of Crystal Falls, Michigan have become quite enamored with the nearby humongous fungus. They now hold an annual "Fungus Fest" every September. You can buy a humongous fungus burger (unfortunately not made with Armillaria, which is in fact a delicious edible mushroom) or fungus fudge (for some reason this does not sound appealing to me...) in their restaurants. Humungus (sic) Fungus t-shirts are available in their stores, but few people from Crystal Falls have ever seen the humongous fungus or could identify its fruiting bodies. In fact, the picture on their humongous fungus web page (http://www.crystalfalls.org/humongou.htm - DEAD LINK) is clearly a Leccinum, a bolete. Yes, I have told them about it, and yes, I offered them the use of one of my pictures of Armillaria. I tried.

The humongous fungus has been great publicity for the science of Mycology; we couldn't buy publicity like this. The humongous fungus even made David Letterman's Top 10 list. (see www.crystalfalls.org).
U-Haul, known for their truck rental services, got into the act in about 1997, when they contacted me for more information about the humongous fungus. Famous for publicizing some of the more bizarre "roadside attractions" on their trucks, U-Haul planned on putting the humongous fungus on some of their trucks to honor the state of Michigan. Through my web page, they contacted me and asked to use one of my pictures. I consented, hoping to help promote mycology to the masses. A month later they sent me a sample drawing for my approval-and the mushrooms were PINK! I diplomatically pointed out that in fact the mushrooms were not pink and that they should put them on the truck in their natural tan/brown/yellow color, since there were thousands of professional and amateur mycologists throughout North America who would know that their fungi were discolored. The U-Haul people replied back that they had taken some "artistic license" with the color, since they thought the natural color was not exciting enough. Sheesh. So now there are several hundred U-Haul trucks around the continent with pink Armillaria fruiting bodies on them. U-Haul now even has a website about the humongous fungus (Anonymous 2002A). It's exciting for me to see one of my pictures (well, sort of one of my pictures...) on one of the 500 or so Humongous Fungus trucks as I drive down the highway-- I've seen the humongous fungus trucks from Maine to California, from Minneapolis to Houston. I like to think it's helping to make the public more aware of fungi and mycology. Myron Smith again wrote: "I like to think that what grabbed the imagination of the public in this case was the idea that there are common, unseen things all around us that are magnificent. Of course, the mental image of a large, old fungus lumbering over the countryside is also bizarre and wonderful."
The humongous fungus continues to be a great boon for educating non-mycologists on the importance of fungi in their lives. The important publicity generated by the work of Myron Smith, Johann Bruhn, Jim Anderson, and the others that followed continues to speak well for the science of Mycology. Even more humongous fungi will no doubt continue to be found. As mycologists we have a multitude of Armillaria researchers to thank for putting mycology in the news in favorable light for a very long period of time. Mycology is not likely to get such great publicity again in our lifetimes. But you never know...
Acknowledgments: Thanks to Myron Smith, Jim Anderson, Dan Czederpiltz, and Sean Westmoreland for reading the manuscript and making helpful suggestions.
References
- Anderson JB, Ullrich RC (1979) Biological species of Armillaria in North America. Mycologia 71: 402?414
- Anderson JB, Korhonen K, Ullrich RC (1980) Relationships between European and North American biological species of Armillaria mellea. Exper. Mycol. 4: 87?95.
- Anonymous. 2002A.U-Haul Humongous fungus web page http://www.uhaul.com/supergraphics/fungus/ - DEAD LINK
- Anonymous. 2002B. Crystal Falls Fungus Fest
webpages. DEAD LINK
DEAD LINK - Antonín V (1986) Studies in annulate species of Armillaria - I Study of type-specimens of Armillaria cepaestipes Velenovský. Ceská Mykol. (Praha) 40: 38-40.
- Antonín V (1990) Studies in annulate species of Armillaria - III. Species described by Josef Velenovský. Acta Mus. Moraviae Sci. Nat. 75: 129-132.
- Barla G (1887) Bull. Soc. Mycol. France 3:143
- Dodge SR (2001) An Even More Humongous Fungus, LINK
- Bollock J (1992) Huge organisms. New Scientist 30 May, p. 54
- Gillet C-C (1874) Les Hymenomycètes ou description de tous les champignons (Fungi) qui croissent en France.
- Gould SJ (1992) A humongous Fungus Among Us. Natural History, July p. 10-14.
- Herink J (1973) Taxonomie Václavky Obecné? Armillaria mellea (Vahl. ex Fr.) Kumm. pp. 21?48. In: Vysoká Skola Zem_d_lska V Brné. Vyznamenaná Rádem Prace BRNO; J. Hasek, ed. Brno: Lesnicka fakulta VSZ.
- Hintikka V (1973) A note on the polarity of Armillariella mellea. Karstenia 13:32?39.
- Marxmüller H (1992) Some notes on the Taxonomy and Nomenclature of five European Armillaria species. Mycotaxon 44 (2): 267-274.
- Marxmüller H, Romagnesi H (1987) Bull. Soc. Mycol. France 103:152
- Shaw CT, Kile GA (1991) Armillaria root disease. Agriculture handbook 691, 233 pp.
- Smith M, Bruhn J, Anderson J (1992) The fungus Armillaria bulbosa is among the largest and oldest living organisms. Nature 356:428-431
- Smith ML, Duchesne LC, Bruhn JN, Anderson JB (1990) Mitochondrial genetics in a natural population of the plant pathogen Armillaria. Genetics 126: 575-582
- Termorshuizen A, Arnolds E (1987) On the nomenclature of the European species of the Armillaria mellea group. Mycotaxon 30: 101-116.
- Velenovský J (1920) Ceské Houby 1: 1-950
- Velenovský J (1927) Václavka hlíznatá (Armillaria bulbosa Barla) Mykologia (Praha) 4: 116-117; translation to English by Vladimír Antonín. (Pers. Comm.)
- Volk TJ, Burdsall HH Jr. (1995) Nomenclatural study of Armillaria and Armillariella species (Basidiomycotina, Tricholomataceae) Fungiflora, Oslo, Norway: Synopsis Fungorum 8, 121 pp. ISBN 82-90724-14-4
- Volk TJ (1995-2002) Tom Volk's fungi. TomVolkFungi.net
Of course, you can't do fungal ecology unless you know what fungi are present. There is almost certainly no habitat in the world whose fungi have been fully enumerated. A group of 22 mycologists gathered in Costa Rica in 1995 and came up with a strategy for isolating and identifying all of the fungi (an estimated 50,000) in a particular habitat (the Guanacaste Conservation Area) -- an All-Taxa Biodiversity Inventory for fungi (Rossman et al. [eds.] 1998 -- see reference below)This ambitious plan called for a staff of 100, $1 million worth of agar media, 1.8 million slants to isolate the endophytic fungi alone... Unfortunately all this would have cost about US$25 million, so it hasn't been done. But the need remains, and the general lack of knowledge about fungi means that they are not usually considered when conservation issues are raised. Perhaps you can help to change that.
A less ambitious ATBI is now under
way in Great Smoky Mountains National Park, but it is a long-term
endeavour -- visit the web site at http://www.discoverlife.org/
As of September 2003, about 2,200 fungi had been recorded for the
Smokies ATBI. This is hundreds more than were known from this area
prior to the study, and the number is expected to rise inexorably as the
study continues...
A well-known and
scientifically ambitious couple, Oluna and Adolf Ceska, living in
Victoria, BC, began in 2004 to compile an inventory of the fungi of
Observatory Hill, a largely forested area, 71.4 ha in area and 224m
high, just north of Victoria. After several years, and by dint of making
almost 200 trips to the hill, they have as of late 2012 collected more
than 1,100 species, and the study continues... They have found a number of
extremely rare species, and some undescribed taxa. Theirs is now
probably the most intensive and species-rich study for such a small area
in the history of mycology. An account of the study can be accessed
here and
here
During the same years the Ceskas also participated in a multi-year study of Clayoquot Sound, including Pacific Rim National Park, other team members being Christine Roberts, Paul Kroeger and Bryce Kendrick (Roberts et al. 2004). They found 551 species, only 28 being collected in all years, and 308 species being found in only one year.
A similar study in the Haida Gwaii archipelago, with the same 5 mycologists, visiting the islands 2 or 3 times per year, found over 600 taxa, and led to the publication of a book 'The Outer Spores - Mushrooms of Haida Gwaii' (Kroeger et al. 2012).
Now we can approach this problem in a completely new way, as this article in PNAS describes:
... researchers traveled to 26 pine forests across North America and collected 10-cm-deep soil cores, more than 600 in all. Within hours of collection, and with the assistance of local scientists and universities, they preserved the samples to extract and isolate the fungal DNA. The researchers then used modern genomic tools to sequence unique stretches of the environmental DNA that can be used as barcodes to identify all of the fungal species present in each sample.
The sequencing revealed more than 10,000 species of fungi, which the researchers then analyzed to determine biodiversity, distribution, and function by geographical location and soil depth. Interestingly, author Peay said, there was very little overlap in the fungal species from region to region; East Coast fungi didn't show up on the West Coast or Midwest, and vice versa.
"People often assume that similar habitats in, say, North Carolina and California would have similar fungi, but this is the opposite of what we find," an author said. "What’s more interesting, despite the fact that soil fungal communities in Florida and Alaska might have no fungi in common, you find that many of the processes and the functional rates are convergent. The same jobs exist, just different species are doing them."
The team found this to be particularly true when comparing the functionality of fungi at different strata of the core samples. Even though the samples were collected thousands of miles apart, fungi near the top all performed the same task; similarly, bottom fungi performed very similar functions across the continent.
We need many more similar studies to establish baseline data for North American fungi, such as have existed for many years in Europe, which may give us warnings about decline and loss of species resulting from anthropogenic influences (habitat loss, climate change).
And having outlined that encouraging state of affairs, we must turn the page to another,
completely different aspect of mycology which came
to prominence in the middle of the 19th century, and has remained front
and centre ever since...
Go to Chapter 12
Go to Table of Contents
© Mycologue publications 2020
Further Reading on Fungal Ecology
Baerlocher F, Kendrick B (1981) The role of aquatic hyphomycetes in the trophic structure of streams. pp. 743-760 (in) The Fungal Community: its Organization and Role in the Ecosystem. (eds.) E.T. Wicklow ET, Carroll GC. Marcel Dekker, New York.
Bell A (1983) Dung Fungi: an illustrated guide to coprophilous fungi in New Zealand. Victoria University Press, Wellington.
Cannon PF (1995) An ATBI - How to find one and what to do with it. Inoculum 46: 1-4
Deighton J (2003) Fungi in Ecosystem Processes. Mycology Series No. 17. 424pp Marcel Dekker.
Doveri F (2004) Fungi Fimicoli Italici: A guide to the recognition of basidiomycetes and ascomycetes living on faecal material. 1104 pp. Assoc. Micol. Besadola
Frankland JC (1998) Fungal succession - unravelling the unpredictable. Mycol. Res. 102: 1-15
Hudson HJ (1980) Fungal Saprophytism. 2nd Edn. Arnold, London.
Ingold CT (1966) The tetraradiate aquatic fungal spore. Mycologia 58: 43-56.
Ingold CT (1975) Guide to Aquatic Hyphomycetes. Freshwater Biological Assoc. Publ. # 30. 96 pp. Ambleside.
Kendrick B (1958) Microfungi in pine litter. Nature
181: 432.
Kendrick B (1958) Helicoma monospora sp. nov. from pine litter.
Trans. Brit. mycol. Soc. 41: 446-448. [later made the type species of
Slimacomyces
Minter]
Kendrick B (1958) Sympodiella, a new hyphomycete genus.
Trans. Brit. mycol. Soc. 41: 519-521.
Kendrick B (1959) The time factor in the decomposition of coniferous leaf litter.
Can. J. Bot. 37: 907-912.
Kendrick B (1961) Hyphomycetes of conifer leaf litter. Thysanophora gen. nov.
Can. J. Bot. 39: 817-832.
Kendrick B, Burges A (1962) Biological aspects of the decay of Pinus sylvestris
leaf litter. Nova Hedwigia 4: 313-342.
Kroeger P, Kendrick B, Ceska O, Roberts C (2012) The Outer Spores: Mushrooms of Haida Gwaii. Mycologue Publications, Sidney, BC. and Haida Gwaii Museum, Skidegate. http://www.mycolog.com/mycologueproducts.html
Michaelides J, Kendrick B (1982) The bubble-trap propagules of Beverwykella, Helicoon and other aero-aquatic fungi. Mycotaxon 14: 247-260.
Nikolcheva LG, Bourque T, Baerlocher F (2005) Fungal diversity during initial stages of leaf decomposition in a stream. Mycol. Res. 109: 246-253.
Paulus B, Gadek P, Hyde KD (2003) Estimation of fungal biodiversity in tropical rainforest leaf litter using particle filtration: the effects of leaf storage and surface treatment. Mycol. Res. 107: 748-756.
Peabody RB, Peabody DC, Sicard KM (2000) A genetic mosaic in the fruiting stage of Armillaria gallica. Fungal Genetics and Biology 29: 72-80.
Peabody RB, Peabody DC, Tyrrell MG, Edenburn-MacQueen E, Howdy RP, Semelrath KM (2005) Haploid vegetative mycelia of Armillaria gallica show among-cell-line variation for growth and phenotypic plasticity. Mycologia 97: 777-787.
Price PW (1988) An overview of organismal interactions in ecosystems in evolutionary and ecological time. Agriculture, Ecosystems and Environment 24: 369-377.
Richardson MJ, Watling R (1982) Keys to fungi on dung (Revised Edition). British Mycological Society, Cambridge.
Richardson MJ (2001) Diversity and occurrence of coprophilous fungi. Mycol. Res. 105: 387-402.
Roberts C, Ceska O, Kroeger P, Kendrick B (2004) Macrofungi from six habitats over five years in Clayoquot Sound, Vancouver Island. Can. J. Bot. 82: 1518-1538.
